-
 CLINICAL RESEARCH TECHNOLOGY (CRO)FOCUSED ON CLINICAL TRIALS MANAGEMENT ACCORDING TO ICH/GCP STANDARD
CLINICAL RESEARCH TECHNOLOGY (CRO)FOCUSED ON CLINICAL TRIALS MANAGEMENT ACCORDING TO ICH/GCP STANDARD -
 A STRONG SCIENTIFIC APPROACH, HIGHLY QUALIFIED PERSONNEL AND TECHNOLOGICALLY ADVANCED SOLUTIONS
A STRONG SCIENTIFIC APPROACH, HIGHLY QUALIFIED PERSONNEL AND TECHNOLOGICALLY ADVANCED SOLUTIONS
Enstablished in 1999, as full-service Contract Research Organization focused on managing clinical trials according to the standards of Good Clinical Practice (ICH-GCP) through an extensive use of new technologies.
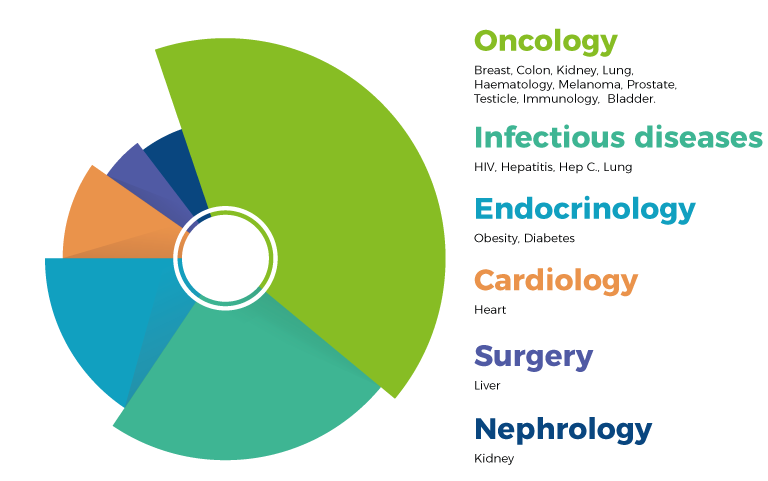
CRT company is specialized in the design, implementation and management of clinical trials (I, II, III and IV phase), observational studies, medical devices, epidemiological and drug-economy trials for the development and recording of new drugs or products , new therapeutic indications of existing drugs or products, and new therapeutic regimens. The number of managed projects increased steadily over the years, covering different therapeutic areas including oncology, cardiology, infectious diseases, nephrology and surgery.

MEDICAL WRITING
Clinical Protocol development (UNI EN ISO 14155: 2020 and ICH-GCP)
Clinical Study Report development (ICH-GCP)
Informed Consent Form development
Documents for Investigator's Meetings (abstracts, posters, presentations)
Literature Research
SITE SELECTION AND FEASIBILITY
Adherence to the project and to the needs of the Sponsor
Coverage of different therapeutic areas
Adequacy of recruitment potential
Staff adequacy, extensive experience
Data Quality level adequacy

REGULATORY AFFAIRS
Development of regulatory documents for ECs and CAs
Study submission to ECs and CAs
Negotiation and finalization of financial agreements
Obtaining ECs opinions and CAs authorizations
Periodic notification to ECs and CAs
PROJECT MANAGEMENT
Flexible budget models
Identifying winning strategies and creating appropriate timelines
Identification and timely resolution of problems
Efficiency in communication processes between all parties
Constant reporting

MONITORING
Feasibility, start-up, routine, unscheduled and close-out visits
Expedited planning and preparation of visits
Development of Visit Reports to Sponsor and Follow-up letters to Investigators
Certified and experienced CRA
Development of Clinical Monitoring Plan based on risk assessment
DATA MANAGEMENT
Data Management Plan development
Case Report Form (CRF) development and eCRF implementation (CFR 21 part 11 compliant)
Clinical Database development and maintenance
Validation of data through edit checks and ad hoc electronic tools
Data encoding (Adverse Events and Concomitant Treatments)
Import of clinical data and data transfer

BIOSTATISTICS
Input to Clinical Study Protocol
Development of the Statistical Analysis Plan (SAP)
Development of the randomization list
Tables, Listing and Figures (TLF)
Interim and final statistical analysis
Management of Data Monitoring Boards (DMBs)
Statistical advisory

PHARMACOVIGILANCE
Receipt and review of SAE reports (initial and follow-up)
Recovery of missing information and requests for clarification
Transmission of SAE reports (initial and follow-up) to the PV office of the Sponsor
Recording of SAEs in the safety database and encoding of terms
Development and submission of periodic safety reports
SAEs reconciliationi
CRT is accredited to the Italian Medicines Agency (AIFA) as CRO according to D.M. 15/11/2011 and ISO 9001: 2015 certified (Certificate N ° AJAEU / 08/10434) on the following activities:
“Design and supply of clinical and epidemiological trial services, design and development of technological solutions for collection and management of clinical data;collection services provision, management and analysis of data related to clinical and epidemiological trials “.
WHO WE ARE
Demonstrating that the use of innovative technological tools, in the managing and conduct of clinical trials, allows to an improvement of the results reducing time and costs.
eClinical is a web-based platform internally developed by CRT, according to the US Computer System Validation (US FDA – 21 CFR Part 11: Electronic Records; Electronic Signatures). Therefore, this software is able to ensure the highest standards in terms of security and quality of collected data.
Moreover, since it is based on web technology, it doesn’t need any installation and can be used through normal browsers (Chrome, Explorer, Firefox).
Over 10,000 patients have been enrolled through eClinical over the past two years.

